Abstract
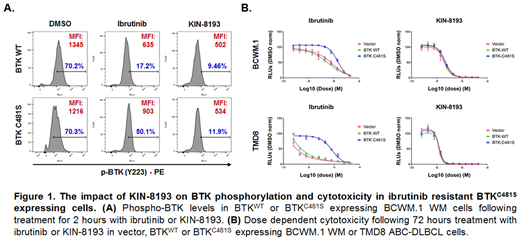
Activating mutations in MYD88 alone or in coordination with BCR activating mutations transactivate Bruton's tyrosine kinase (BTK) in WM and ABC DLBCL cells (Yang et al, Blood 2013; Wilson WH et al, Nat Med 2015; Phelan JD, Nature 2018). Ibrutinib is a covalent inhibitor that binds to BTKCys481 and is active in MYD88 mutated WM and ABC-DLBCL (Treon et al, NEJM 2015; Wilson et al, Nat Med, 2015). Acquired resistance to ibrutinib is increasingly being recognized in WM, as well as other B-cell malignancies due to somatic mutations at BTKCys481 that abrogate BTK-ibrutinib binding (Xu et al, Blood 2017). BTKCys481 mutations are usually sub-clonal, but can drive ibrutinib resistance and protect BTK wild-type clones through a paracrine mechanism involving activation of ERK1/2 (Chen et al, Blood 2018). Hematopoietic cell kinase (HCK) which is aberrantly upregulated and transactivated by mutated MYD88, and triggers multiple pro-survival signaling cascades that include activation of BTK, as well as PI3K/AKT and MAPK/ERK1/2 (Yang G et al, Blood 2016). We therefore examined if inhibition of HCK could abrogate BTK activity and overcome ibrutinib resistance driven by BTKCys481 mutations in MYD88 mutated B-cell lymphomas. We performed a screen of 220 clinical and preclinical kinase inhibitors to identify compounds with potent HCK inhibition. Over 100 analogs of three series of promising compounds with HCK activity were synthesized and triaged. Target deconvolution was performed to clarify selectivity, and other important kinase targets. These efforts led to the selection of a lead compound identified as a type-II kinase inhibitor, KIN-8193, with a molecular weight around 600. Single-digit nanomolar (nM) biochemical and double-digit nM cellular potency was demonstrated, with high selectivity (S-score 0.07) in line with that observed with ibrutinib (S-score of 0.03). Live-cell target engagement for HCK was confirmed by competitive ATP-biotin binding assay. DMPK and PK studies showed very high levels of mouse, rat, and human microsomal stability (42.4, 60.2 and 79.3 minutes respectively), and oral bioavailability in mice (48%) and rats (79%). Cmax reached 2.0 µM, while T1/2 was 26.8 hours with 25 mg/kg single oral dosing in rats. Rats toxicology studies showed excellent tolerability in 28-days repeated oral dosing with 25 mg/kg/biw dosing schedule. No relevant inhibition was observed against a panel of 100 other receptor targets, including hERG. AMES was negative up to 100 uM, and Cyp inhibition studies showed acceptable inhibition up to 10 uM. KIN-8193 potently inhibited the phosphorylation of HCK(p-Y411) and its downstream target BTK(p-Y223) in both BTK wild-type and ibrutinib-resistant BTKCys481 mutated WM and ABC DLBCL cell lines driven by activating MYD88 mutations, and primary WM cells (Fig 1A). Target engagement studies showed HCK but not BTK direct binding. In vivo pharmacodynamic (PD) studies using luciferized BCWM.1 cells WM xenograft mouse model showed potent dose-dependent inhibition of HCK(p-Y411) and BTK(p-Y223) at 6 and 24 hours following single dose administration of KIN-8193. Importantly, KIN-8193 showed selective cytotoxicity against MYD88 mutated BTK wild-type and BTKCys481 mutated, ibrutinib-resistant WM and ABC DLBCL cell lines, and primary WM cells, but had no impact on healthy donor B- and T-cells at pharmacologically achievable levels (Fig. 1B). We therefore describe a novel, highly selective and potent HCK inhibitor that is well tolerated in long-term rat toxicology studies and shows selective killing of MYD88 mutated WM and ABC DLBCL cells. Inhibition of HCK by KIN-8193 blocks downstream wild-type BTK and Cys481 mutated BTK activity, and overcomes ibrutinib resistance induced by BTKCys481 mutations.
Hunter:Pharmacyclics: Consultancy. Castillo:Genentech: Consultancy; Janssen: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Beigene: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Millennium: Research Funding. Gray:Syros, Soltego, Petra, C4 Therapeutics: Equity Ownership. Treon:Pharmacyclics: Consultancy, Other: Travel, Accommodations, Expenses, Research Funding; BMS: Research Funding; Johnson & Johnson: Consultancy; Janssen: Consultancy, Other: Travel, Accommodations, Expenses.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal